FATTY ACIDS AND DIABETES PREVENTION

![]()
Metabolic syndrome
Chronic non-communicable diseases such as heart disease, cancer and diabetes are the leading causes of mortality in the world. The common denominator seems to be the “Western” diet style, made of food with poor quality ingredients and from chemical or unsustainable processes, which has dramatically increased the incidence of metabolic dysfunctions [1]. Although we are led to think that overweight and obesity lead to chronic diseases, in fact many normal-weight people already have symptoms that can be linked to metabolic syndrome: dyslipidemia, hypertension, hyperglycemia, insulin resistance and nonalcoholic hepatic steatosis.
Insulin resistance
There is now ample evidence from epidemiological studies that excessive sugar consumption may be a common condition for the symptoms of metabolic syndrome, such as hypertension, hypertriglyceridemia, hepatic steatosis and insulin resistance.
Excessive and prolonged blood glucose levels lead to a reduction in tissue sensitivity to insulin, causing hypersecretion of insulin by the pancreas in an attempt to maintain glucose homeostasis (compensatory hyperinsulinemia). This condition eventually results in the “exhaustion” of pancreatic beta cells, that reduce insulin secretion leading, eventually, to the onset of diabetes.
The most significant metabolic abnormalities are observed in tissues sensitive to insulin stimulation: adipose tissue, liver and muscle.
- in normal conditions, adipose tissue is responsible for the accumulation of intracellular lipid droplets, from which energy is obtained by mobilization following stimulation given by insulin. But when the adipocyte cell exceeds the physiological limit of intracellular fat accumulation, its fate is to either become dysfunctional, ceasing to produce insulin-sensitizing adipokines (such as adiponectin) and further promoting insulin resistance of other tissues, or to go into programmed death and inducing an immune response.
- in normal conditions, muscle is responsible for the uptake of up to 80% of postprandial glucose. On the other hand, in cases of peripheral (muscle) insulin resistance, plasma glucose homeostasis deteriorates sharply. The situation is further aggravated by the increased availability of fatty acids released into the circulation from dysfunctional adipose tissue. While initially the muscle can use these fatty acids for energy purposes, in the long run muscle cells lose this metabolic flexibility, beta-oxidation of fatty acids becomes inefficient and lipotoxic species are formed, further worsening muscle metabolism.
- in physiological conditions, about 60% of fatty acids reaching the liver are derived from adipose tissue. In the case of adipocyte dysfunction, there is an overload of fatty acids that are accumulated as triglycerides, creating excessive storage known as steatosis. Partial triglyceride oxidation and increased formation of the most triglyceride-rich lipid aggregations, such as LDL and VLDL responsible for hypertriglyceridemia with unfavorable outcomes, will occur.
Overall, the onset of insulin resistance in insulin-sensitive target tissues causes major metabolic abnormalities, such as hyperglycemia and hypertriglyceridemia, which are common features of the metabolic syndrome.
Insulin resistance and cell membrane
Extensive experimental evidence suggests a close association between insulin sensitivity and cell membrane lipid composition.
Since insulin signaling occurs by interaction of insulin with its receptor located on the cell membrane, the composition of membrane fatty acids and the physicochemical properties they determine are responsible for modulating the insulin signaling pathway.
There are two main aspects that relate membrane composition to insulin resistance:
1) NUMBER AND AFFINITY OF INSULIN RECEPTORS
Decreased membrane fluidity due to increased saturated fatty acid (SFA) content in phospholipids has been shown to prevent the proper assembly of the insulin signaling protein apparatus on the cell surface due to limited protein mobility in the lipid bilayer.
2) PLASMA GLUCOSE UPTAKE.
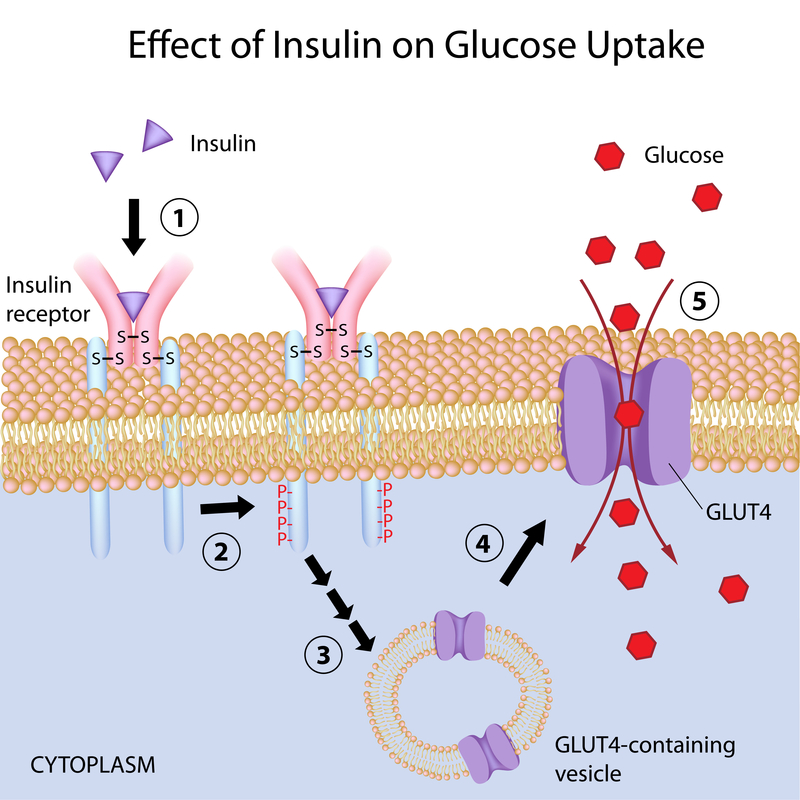
Glucose enters cells through specific transporter proteins called GLUTs (glucose transporters). GLUT4, found in adipocytes and muscle cells, differs from the other transporters of the same family in that about 90% is stored in intracellular vesicles that are mobilized only when insulin binds the receptor on the plasma membrane. At that point, GLUT4-containing vesicles migrate to and merge with cell membrane. Once localized on the plasma membrane, GLUT4 transporters subsequently facilitate the entry of plasma glucose into the cell.
This transport mechanism across membranes can be strongly influenced by membrane fluidity. Indeed, decreased membrane fluidity has been shown to hinder vesicle fusion with the plasma membrane and the exposure of GLUT4 on the cell surface, thereby impairing the insulin sensitivity of muscle and adipocyte cells.
Dietary fat and insulin resistance
Studies in animal models and humans have accumulated an important body of evidence establishing the connection between dietary fat, membrane lipid profiles and insulin resistance (Membrane Theory of Diabetes [2]). The quality and quantity of dietary fat intake may, in fact, promote or counteract the onset of insulin resistance through incorporation into cell membranes and maintenance of membrane structure.

Balanced dietary fat intake are crucial in restoring and maintaining insulin sensitivity, especially considering that metabolic dysfunction in target tissues (as we have seen: adipose tissue, muscle, and liver) begins to occur decades before the diagnosis of type 2 diabetes.
– dietary consumption of saturated SFA and trans fatty acids changes the composition of the cell membrane leading to an increase in the stiffness of the lipid bilayer. This can hamper GLUT4 exposure and insulin receptor function in target tissues. In addition, SFAs tend to accumulate in intracellular deposits that are difficult to mobilize. When used as an energy source, they are partially oxidized in the mitochondria, thereby promoting the formation of reactive oxygen species, lipotoxic products and impaired muscle and liver cell function. The same applies to carbohydrate-rich diets since glucose is rapidly converted to SFA (palmitic acid, 16:0) through de novo lipogenesis in the liver and in adipocytes.
– Replacement of saturated with monounsaturated fatty acids (MUFA) in the diet improves insulin sensitivity in both healthy and diabetic subjects. Extra virgin olive oil, as the main source of MUFA (oleic acid) in our diet, results in better glycemic control and improved insulin-sensitivity in patients as compared to those who mainly use sunflower oil (rich in omega-6 PUFAs). In addition, MUFA fatty acids are more efficiently oxidized in the mitochondria to produce energy and, at the same time, promote the complete oxidation of SFA acids, thus preventing the formation of free radicals.
– Diets with a low omega-6/omega-3 ratio have been shown to be effective in counteracting adipocyte and muscle insulin resistance. This is due to both increased membrane fluidity by incorporation of high unsaturated omega-3 polyunsaturated fatty acids (PUFA). In addition, the insulin-sensitizing effect also occurs through enhancing GLUT4 and insulin receptor gene expression through the binding of specific transcription factors.
Article by the Editorial Group of Lipinutragen
The food recommendations in the article are not intended as a substitute for a personalized meal plan and are to be adapted to specific cases.
Bibliographical references:
[1] “Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017” DOI: https://doi.org/10.1016/S0140-6736(19)30041-8
[2] Pilon M. “Revisiting the membrane-centric view of diabetes” Lipids in Health and Disease (2016) 15:167.
Learn more:
Photo: 123RF Archivio Fotografico: 126263301 : ©artinspiring | 70028082 : @moremar
- On 9 November 2022