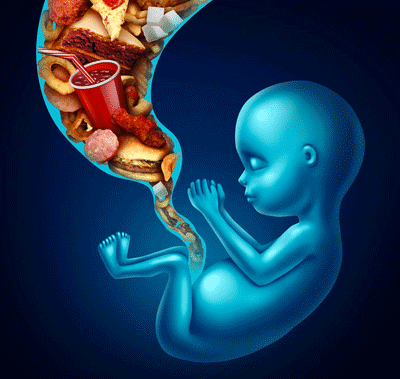
Childhood obesity
Nutrition education begins in the womb
Our time being is when the word “pandemic” has become in everyday use. And yet, the same term has extended been used to refer to those metabolic pathologies, typical of adulthood, which today are increasingly affecting children as well. Diagnosis of obesity, diabetes, fatty liver disease in pediatric age are on the rise with an alarming trend.
Although most of the interest is oriented to the environmental and behavioral factors that influence the health of the child in his first years of life (including the excessive consumption of high-calorie foods with low nutritional content and sedentary habits), the scientific evidence points the attention also to the so-called metabolic programming in utero which contributes to determining the behavior of the unborn child towards food.
The APPETITE REGULATION
The regulation of food intake is the result of a complex neuronal communication network that occurs mainly in the hypothalamus, where the behavior of each individual towards food is defined.
During fetal development, “appetite neurons” (orexigens) and “satiety neurons” (anorexigens) are formed. These, in numerical balance with each other and in close connection with the peripheral organs responsible for glucose and lipid metabolism, will regulate food intake based on the caloric and nutritional requirements of the organism of the unborn child.
Beside the control of the energy balance, the neuronal reward circuit is also formed in the hypothalamus, mediated by the action of dopamine, thanks to which the individual will be “rewarded” with pleasure after having performed a function necessary for his survival (such as, in fact, that of eating) and will be induced to repeat the action.
Homeostatic hunger and hedonic hunger
Our approach to food is therefore defined by both the energy levels and the pleasure levels that the brain uses as sensors to determine when to resort to food consumption.
Homeostatic hunger is activated following an energy drop: food intake is sought and then stopped when the required nutrient and energy intake has been reached.
In addition, for the survival of the species, we have also developed the so-called hedonic hunger: the pleasure linked to the consumption of food (especially the one with the highest energy intake) induces to continue to introduce food, even when there is no need for energy.
In humans, the neuronal centers of homeostatic hunger and those of hedonic hunger are closely associated so that the hedonic hunger is regulated by the homeostatic one according to the real needs of the organism.
Plan on obesity
According to the concept of metabolic programming in utero, the nutritional and hormonal environment of the mother influences the development of the organs responsible for managing the energy balance (brain, adipose tissue, pancreas, liver, muscle …), determining the ability to regulate metabolism of the child in the years to come.
In humans, neurogenesis and the formation of neuronal circuits responsible for regulating appetite occur almost entirely during fetal life. It is therefore extremely important to control the diet and metabolic condition of the pregnant woman since the nutrients introduced in pregnancy and the circulating hormones could alter the baby’s neuronal programming and therefore the behavior it will have towards food throughout its life.
The scientific literature on the subject is vast and any review is highly reductive. However, to summarize, the main nutritional or hormonal stressors during pregnancy, which have a negative impact on metabolic imprinting, are:
- state of overweight or obesity of the mother at the time of conception
- rapid and marked weight gain during pregnancy
- high-fat diet (see paragraph “the role of fats”)
- pre-existing condition of insulin resistance or diabetes in the pregnant woman
Specifically, but not exhaustively, with regard to the development of the neuronal centers of appetite regulation, we mention the following effects.
- A diet high in saturated fat or exposure to a hyperglycemic condition
- decreases the number of neurons that are formed
- it alters the “satiety neurons” / “appetite neurons” balance in favor of the latter
increasing the preference for high-fat foods and promoting impulsive behavior towards food.
- A diet rich in junk food during pregnancy directs the unborn child’s preference towards junk foods, which are more palatable, fatter, sweeter or more salty, inducing a sort of saturation (burnout) of the reward system that is satisfied only with more appetizing foods.
- The dysfunctional adipose tissue of the obese mother subjects the fetus to excessive exposure to leptin and insulin resulting in
- alteration of neurogenesis (number of neurons formed)
- alteration of the organization of the synapses in the hypothalamus
- selective inhibition of satiety neurons
predisposing the unborn child to develop an impulsive and hyperphagic behavior.
- High levels of inflammatory cytokines related to the inflammatory condition of the obese mother
- disrupt dopaminergic reward systems
- disturb the serotonin systems of mood
producing states of anxiety accompanied by hyperphagic behavior in the child, in a way that is completely comparable to states of drug addiction (“addition-like eating”)
All these effects on eating behavior have the potential to be perpetuated in subsequent generations when girls, born with this type of metabolic imprinting, are in turn pregnant with metabolic problems and / or strongly altered eating habits.
The role of fats
Following the publication in the scientific journal Lancet of David Barker’s hypothesis on the origins of health and chronic diseases, many of the studies on animal models aimed at understanding the effects of the nutritional status of the pregnant woman on the metabolic programming of the fetus have focused on fats mainly saturated, without paying attention to the proportion between families of saturated, monounsaturated, polyunsaturated omega-6 and omega-3.
Although it is true that in terms of macronutrients, it is the amount of fat (not protein or carbohydrates) that defines the strongest correlations between maternal diet, metabolic imprinting and infant feeding behavior, today this categorization is conceptually reductive.
The central role of fats in the pregnant woman’s diet, as a determinant of the risk for the baby, is not a surprising concept since it is known how important they are in the creation and functionality of cell membranes and therefore in the response of cells to hormonal signals, also in terms of regulation of the expression of genes responsible for energy metabolism. However, we need to go one step further and differentiate: which fats are we talking about?
We cannot in fact forget that the dramatic increase in the incidence of cardio metabolic diseases of the last 50 years goes hand in hand with the spread of the Western diet, rich in foods processed with vegetable and refined oils. The major imbalances in the fat composition of the modern diet can be summarized as:
– very strong increase in omega-6 sources (linoleic acid from vegetable oils) to the detriment of omega- 3 of animal origin (EPA and DHA) and, consequently, a strong imbalance in the omega-6 / omega-3 ratio
– increased consumption of saturated fats from refined oils and products of animal origin
– strong increase in trans fats in food
Therefore, to fully understand the role of excesses or deficiencies of fats, it is necessary to focus attention on the imbalance in the profile of dietary fatty acids introduced with the diet, rather than on the effect of the absolute excess of a family of these.
Conclusions
The prevention of childhood obesity starts from fetal development, that is when maternal nutrients and hormones influence the formation and programming of the systems responsible for managing the energy balance of the unborn child’s body.
It is therefore a critical period for development in which certain metabolic stressors alter the child’s approach to food, predisposing him to develop obesity, insulin resistance, dyslipidemia and hypertension at a young age.
Good prevention can be done by following the principles of nutrilipidomics right from the preconception period by improving eating habits (balance in the intake of fatty acid families in the diet) and the glucose and lipid metabolism of the future mother.
This intervention will be even more effective if customized to the molecular state of the woman in order to define an individual strategy and thus achieve a balance of health to be perpetuated for generations to come.
To know more:
- Developmental Origins of Adult Health and Disease (DOHaD) hypothesis
Barker DJ et al. “Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales” Lancet 1986, 1: 1077–1081 - Programmazione metabolica
Lucas A. “Programming by early nutrition in man. The childhood environment and adult disease.” Ciba Foundation Symposium 156. G. Bock and J. Whelan. Chichester, John Wiley and Sons 1991, 38–50
Bouret SG. “Nutritional programming of hypothalamic development: critical periods and windows of opportunity” International Journal of Obesity Supplements 2012, 2: S19-S24
Shook LL et al. “Fetal brain and placental programming in maternal obesity: A review of human and animal model studies” Prenatal Diagnosis. 2020, 1–12. - DIETA OCCIDENTALE
Simopoulos AP “Essential fatty acids in health and chronic disease” The American Journal of Clinical Nutrition 1999, 70(suppl): 560S–9S
Wolmarans P. “Background paper on global trends in food production, intake and composition” Annals of Nutrition & Metabolism 2009, 55: 244–272. - NUTRILIPIDOMICA
Chatgilialoglu C and Ferreri C. “Nutrilipidomics: A Tool for Personalized Health” Journal of Glycomics & Lipidomics 2012, 2:3
Ferreri C e Chatgilialoglu C. “Membrane lipidomics for personalized Health” Wiley 2015
Article by the editorial team of Lipinutragen
Photo: 123RF Archivio Fotografico: 59197600 @Win Nondakowit / 123rf.com – 123RF Archivio Fotografico: 64104329 @lightwise / 123rf.com
- On 6 November 2020