
HOW TO MEASURE THE INFLAMMATION

Chronic systemic inflammation
Over the past three decades, the incidence of chronic cardiovascular, respiratory, neurodegenerative, autoimmune, diabetes and cancer diseases in the world population has drastically increased. It has been observed that some social, environmental and lifestyle-related factors can promote a condition of chronic systemic inflammation which, successively, can promote the onset of the aforementioned diseases, becoming the main cause of disability and mortality in the world. More than 50% of all deaths worldwide are imputable to inflammatory diseases [1].
The stresses to which people in the Western world are exposed (low physical activity, nutritional surplus, psycho-emotional stress) create an environment that promotes continuous activation of stress response systems. Excessive and/or inappropriate activity of the immune system can lead to a state of unresolved chronic inflammation, called chronic low-grade inflammation.
The most employed biomarkers to evaluate inflammation
Inflammatory markers, such as cytokines IL-1, IL-6 and TFN-alpha, Reactive Protein C and hs-PCR, are detectable in the blood through laboratory analysis, and if found in excess are related to the condition of the subject, which is for example in an urgent inflammatory phase, or with cardio metabolic risk.
The dosage of these markers represents a measure of some of the molecules involved in the condition of inflammation, thus motivating the intervention with an anti-inflammatory strategy, even of an integrated type, to help “resolve” the condition. The term “resolution” is appropriate in the case of this type of inflammation, because the subject is helped to overcome this state and recover the functionality of the affected tissues by bringing the levels of the markers back to normal.
But in the case of an inflammatory condition that has persisted for some time, these markers do not explain a more complex situation in which the subject may find himself, especially in the case in which the ability to control and adapt is lost and therefore the organism is unable to “Find the way” to recover and resolve the inflammation.
Today, the inflammation is a very clear process, right from the primary causes that occur at the cell level, the true functional unit of tissues and, to completely understand what is happening in an inflammatory condition, it is essential to know and evaluate the molecular profile of the membrane of the subject.
Inflammation starts from the cell membranes
The presence of an excess of inflammatory markers in the blood is the result of “something” happening upstream. To monitor the molecular events underlying the inflammatory activation it is necessary to trace the real target from which they start all the phenomena of stress, adaptation and defense response (primarily inflammation!) which is the cell, to then define the most appropriate anti-inflammatory intervention.
The most modern and appropriate diagnostic tool is the membrane lipidomic analysis. In fact, it is precisely the level of omega-6 polyunsaturated fatty acids (DGLA and arachidonic acid) and omega-3 (EPA and DHA) present in the cell membranes of the immune system that determines the start of the response, with the signaling cascades pro inflammatory and, subsequently, that resolves inflammation, through the production of eicosanoids and other molecules such as protectins, maresins and resolvins [2].
By evaluating the presence in the membrane of these fatty acids and the related lipid indices, it is possible to ascertain, even in advance, what is the preferential response activated by the cell following a stimulus. There are well-known profiles that can lead to problems of control of the inflammatory state, such as the inflammatory-immune profile, which has an excess of arachidonic acid and DGLA deficiency or the inflammatory-deficiency profile with omega-3 deficiency. In such cases, chronic inflammation occurs as a result, and the most appropriate intervention is to rebalance the structure of fatty acids [3] thanks to a personalized intervention (strategy based on nutritional-nutraceuticals) recommended by the lipidomics specialist.
Do not forget that inflammation, both “silent” and “overt” type, in the latter case supported by the inflammatory markers mentioned at the beginning of the article, can accompany both before and after the infection event viral or the event of vaccination, as is well demonstrated in the case of the current pandemic. ONE MORE REASON to investigate the condition of a patient who exhibits inflammatory symptoms [4, 5, 6, 7].
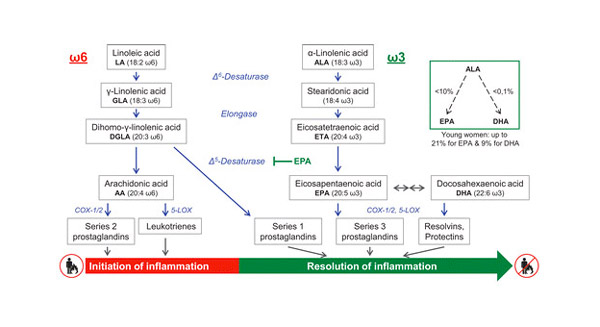
Figure 1 taken from: Whaeler, R. Fatty acids: facts vs fiction. Int J Vitam Nutr Res 2021, 1, 1-21. [3].
Lipidomic analysis is accomplish using the membrane of the mature red blood cell, and is performed in our Lipidomics Laboratory using an accredited robotic procedure. The analysis provides a realistic photograph of the patient’s molecular structure and an assessment – also predictive – of the state of cellular inflammation, useful for the reference professional (pharmacist, doctor or nutritionist) in defining the personalized intervention. Specifically, among the parameters returned by our membrane lipidomic analysis are:
– quantity of linoleic acid, DGLA and arachidonic acid and corresponding indices of enzymatic activity (delta-6 and delta-5 desaturase)
– quantity of EPA and DHA
– omega-6 / omega-3 ratio
– peroxidation index
– PUFA balance, balance index of omega-6 and omega-3 polyunsaturated fatty acids (fundamental indicator for the dosage of these fats).
[1] GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788 (2018).
[2] Serhan, CL. Pro-resolving lipid mediators are leads for resolution physiology. Nature, 2014, 510, 92-101.
[3] Whaeler, R. Fatty acids: facts vs fiction. Int J Vitam Nutr Res 2021, 1, 1-21.
[4] Wong, RSY. Inflammation in COVID-19: from pathogenesis to treatment. Int J Clin Exp Pathol. 2021 Jul 15;14(7):831-844.
[5] Kahn, B. et al. Multisystem Inflammation and Organ Dysfunction After BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccination, Critical Care Explorations: November 2021 – Volume 3 – Issue 11 – p e0578 doi: 10.1097/CCE.0000000000000578.
[6] Khayat-Khoei, M. et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2021 Sep 4:1–14.
[7] Ostrowski, SR. et al. Inflammation and Platelet Activation After COVID-19 Vaccines – Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis. Front. Immunol. 2021, 12:779453. doi: 10.3389/fimmu.2021.779453
The information provided must in no way replace the direct relationship between health professional and patient.
The food recommendations in the article are not intended as a substitute for a personalized meal plan and are to be adapted to specific cases.
- On 24 January 2022



